Penn Quantitative Imaging Resource for Pancreatic Cancer
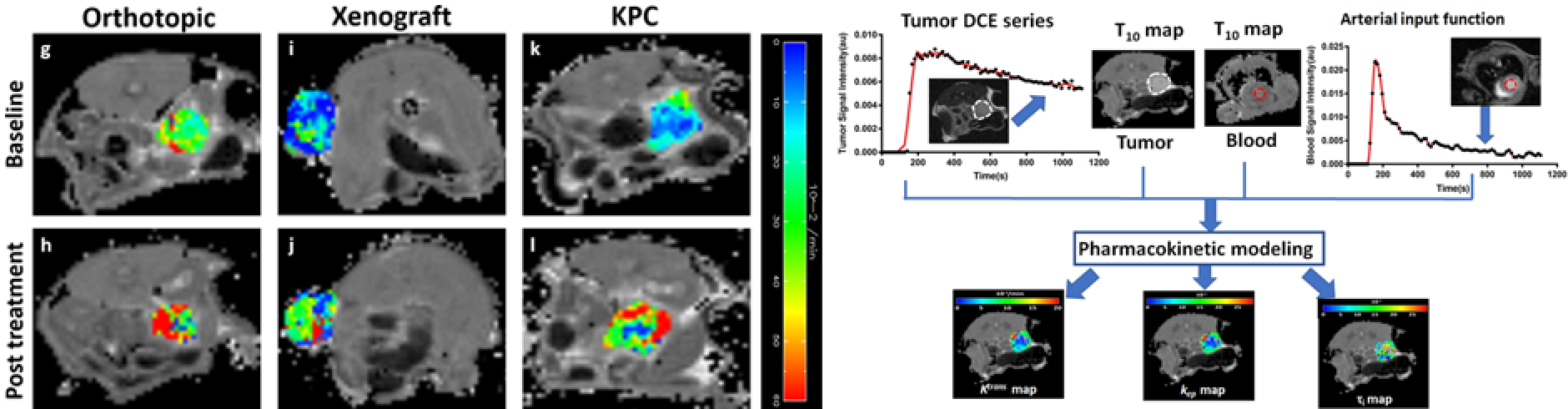

Our mission is to facilitate the development of effective therapies for pancreatic ductal adenocarcinoma (PDA) via the applications of imaging tools. We strive to develop robust imaging tools in murine models of PDA and translate them to the clinic in settings of co-clinical imaging.
Documents describing the procedures for acquiring, processing and analyzing data for pre-clinical and clinical data.
Shared imaging datasets, including both small represenative datasets and full datasets for completed studies.
References and links to abstracts, presentations, and peer-reviewed manuscripts detailing the various aspects of the project.
We will be making updates over the course of the U24CA231858 project (9/2018-8/2023). As the project develops, we will share resources and imaging data produced in the project
Drs Gee and Duda bring in outstanding expertise in informatics and will work on resource/ data sharing aspects of the U24 project while collaborating with team members in cross-modality registration and analysis.
September 1, 2020
University of Pennsylvania Institutional Review Board (IRB) has approved the latest modifications of this trial including the MRI studies which are supported by the U24 resource grant. The MRI protocols on this trial has also been approved by the Center for Magnetic Resonance Imaging & Spectroscopy (CAMRIS).Dr. Peter O’Dwyer, MD is the Principal Investigator of this trial, leading an investigator team from the University of Pennsylvania Abramson Cancer Center, Memorial Sloan Kettering Cancer Center, Salk Institute, and Massachusetts General Hospital. The U24 team members who participate in this clinical study include Drs. Peter O’Dwyer, Thomas Karasic, Mark Rosen, Hee Kwon Song, Rong Zhouand James Gee.
July 23, 2020